23 - Hemmung der Kalkbildung
✏️ Diese Erklärung ist in deiner Sprache noch nicht verfügbar, Hier klicken um deine Übersetzung vorzuschlagen oder schreibe eine E-Mail an fdc.memo@marc-antoinea.fr.
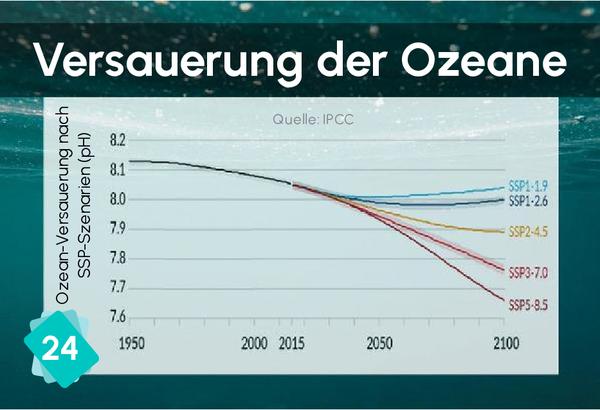
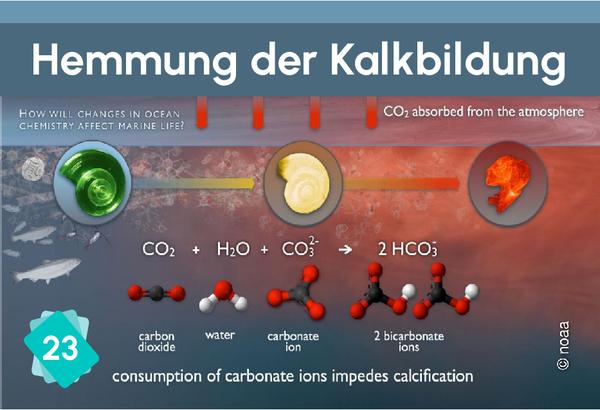
The formation of limestone (calcification) follows the chemical reaction Ca++ + 2HCO3- ⇔ CaCO3 + H2O + CO2. It requires the presence of bicarbonate ions (HCO3-). However, the quantity of these ions in water depends on the pH: in water, carbon dioxide, carbonic acid, bicarbonate ions and carbonate ions are in equilibrium, depending on the pH : CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3- ⇔ 2 H+ + CO32-. The addition of an acid shifts the equilibrium towards the left of the equation. In other words, if the pH drops, there are fewer bicarbonate ions, making it more difficult for organisms to build their shells.